PUBLICATIONS & ABSTRACTS: CLINICAL EVIDENCE
100s of extensive peer reviewed publications and studies utilizing MammaPrint + BluePrint have observed benefits from treatment in the following areas of clinical utility:

Scientific evidence for clinical utility.
By continually expanding and strengthening our database of proven research including 20+ years of clinical validation and 200+ research collaborations, we have gained widespread trust in the precision, accuracy and quality of our MammaPrint® and BluePrint® test suite.
Evidence
Controlling Technical Variation Amongst 6693 Patient Microarrays of the Randomized MINDACT Trial
PUBLICATION: Communications Biology AUTHORS: Laurent Jacob, Anke Witteveen, Inès Beumer, Leonie Delahaye, Diederik Wehkamp, Jeroen van den Akker, Mireille Snel, Bob Chan, Arno Floore, Niels Bakx, Guido Brink, Coralie Poncet, Jan Bogaerts, Mauro Delorenzi, Martine Piccart, Emiel Rutgers, Fatima Cardoso, Terence Speed, Laura van ’t Veer, and Annuska Glas Abstract: Read More
ASCO 2020: Chemokine Signature
PUBLICATION: ASCO 20 AUTHORS: Hatem Soliman, Sangeetha Prabhakaran, Marilin Rosa, Charles Cox, Pat Whitworth, Sahra Uygun, Heather M. Kling, Erin B. Yoder, and William Audeh SUMMARY: Studies demonstrating the presence of immunoregulatory gene activation1 and tumor- infiltrating lymphocytes in the breast tumor microenvironment suggest the importance of an effective anti-tumor Read More
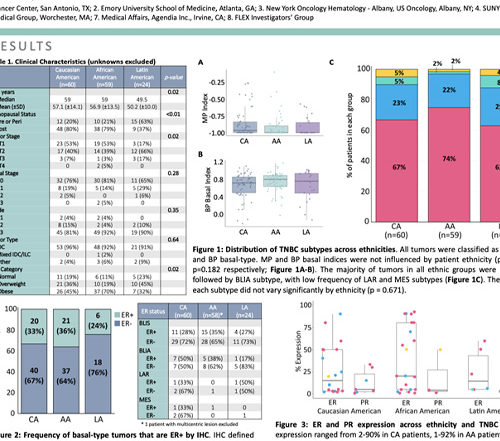
ASCO 2020: TNBC Subtype
PUBLICATION: ASCO 20 AUTHORS: Virginia G. Kaklamani, Cathy Graham, Karen L. Tedesco, Abirami Sivapiragasam, Jennifer Crozier, Apurva N. Shah, Andrea Menicucci, Shiyu Wang, Michelle L. Bolner, Erin Yoder, William Audeh, FLEX Investigators' Group SUMMARY: Triple negative breast cancer (TNBC) is an aggressive histological subtype with few targeted therapies and worse Read More
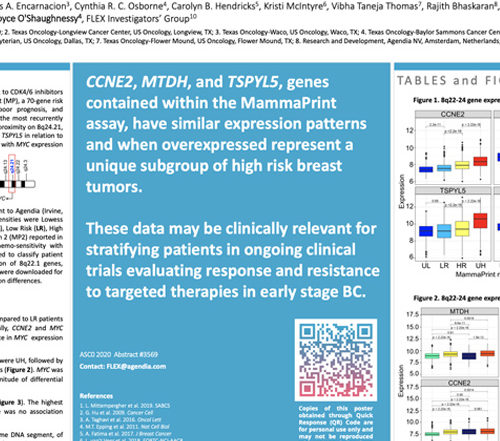
ASCO 2020: 8q22 Poster
PUBLICATION: ASCO 20 AUTHORS: Sami Diab, Matei P. Socoteanu, Carlos A. Encarnacion, Cynthia R. C. Osborne, Carolyn B. Hendricks, Kristi McIntyre, Vibha Taneja Thomas, Rajith Bhaskaran, Josien Haan, Lorenza Mittempergher, Andrea Menicucci, William Audeh, Joyce O'Shaughnessy, FLEX Investigators’ Group SUMMARY: Previous studies have shown that CCNE2 expression is higher in Read More
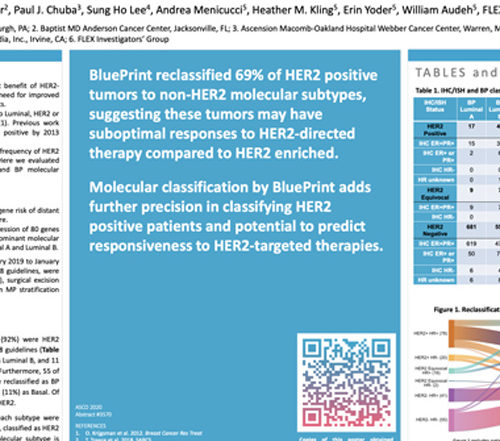
ASCO 2020: HER2 Reclassification
PUBLICATION: ASCO 2020 AUTHORS: Adam Brufsky, Jennifer A. Crozier, Paul J. Chuba, Sung Ho Lee, Andrea Menicucci, Heather M. Kling, Erin Yoder, William Audeh, FLEX Investigators' Group SUMMARY: Biological heterogeneity of HER2 positive breast cancers is supported by a modest benefit of HER2- targeted therapies reported in the APHINITY and Read More
ASCO 2020: FLEX Trials in Progress
PUBLICATION: ASCO 20 AUTHORS: Nina D’Abreo, Jennifer Crozier, Adam Brufsky, Ian Grady, Sami Diab, Blanche Mavromatis, Carrie Dul, Rakhshanda Layeequr Rahman, Laura Lee, Vijayakrishna K. Gadi1, Sarah Untch, Erin Yoder, Heather M. Kling, Amy M. Truitt, William Audeh, Bastiaan van der Baan, FLEX Investigators Group SUMMARY: Genomic expression profiles have Read More
Performance Characteristics of the BluePrint® Breast Cancer Diagnostic Test
PUBLICATION: Translational Oncology Volume 13, Issue 4, April 2020, 100756 AUTHORS: Lorenza Mittempergher, Leonie JMJ Delahaye, Anke T Witteveen, Mireille HJ Snel, Sammy Mee, Bob Y Chan, Christa Dreezen, Naomi Besseling, Ernest JT Luiten, Annuska M Glas SUMMARY: The analytical performance of a multi-gene diagnostic signature depends on many parameters, Read More