OUR TESTS
MammaPrint – Breast Cancer Testing

Will my patient’s breast cancer return after surgery?

Will my patient’s breast cancer return after surgery?
The MammaPrint® test analyzes the 70 most important genes associated with breast cancer recurrence. Results are typically available in 6 days or less, MammaPrint enables quicker, more informed decisions on pre- and post-operative treatment and can easily be integrated into diagnostic workups.1

I see MammaPrint as a great tool which helps you to navigate through difficult decisions.”
— Andrea, a breast cancer survivor
I see MammaPrint as a great tool which helps you to navigate through difficult decisions.”
— Andrea, a breast cancer survivor
BREAST CANCER TREATMENT PLANNING
MammaPrint Test & Results
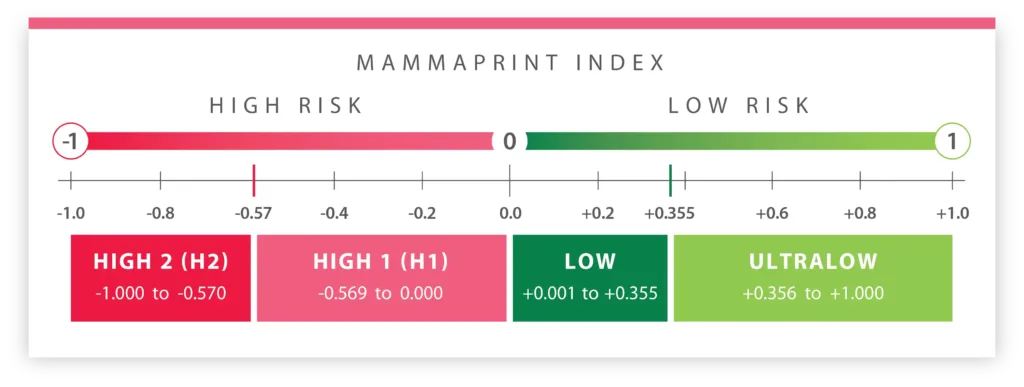
Definitive results for adjuvant chemotherapy planning.
LOW RISK PATIENTS HAVE A
1.3%
CHANCE OF RECURRENCE 2
The MINDACT trial determined these patients do not benefit from chemotherapy.3
HIGH RISK PATIENTS HAVE A
11.7%
CHANCE OF RECURRENCE 2
These patients were shown to have significantly better outcomes with chemotherapy.4
Nearly half of clinically high risk patients were reclassified as MammaPrint Low Risk and were able to forgo chemotherapy without compromising their outcomes.3
OUR PROOF
Implemented across the entire medical community.
MammaPrint is FDA-cleared for women of all ages. MammaPrint is also available via next generation sequencing on Illumina MiSeq platform. This approach has been CE marked allowing use in the European Union.
Supported by
Data
The validity and utility of
MammaPrint is supported by numerous
peer-reviewed publications.
Recommended
by Leading Authorities
Chosen by
Physicians
Healthcare professionals reported higher confidence in their treatment recommendations after receiving a MammaPrint result.
Requested by
Patients
Having learned the unique benefits of
genomic testing in recent years, thousands
of patients globally have requested MammaPrint.
THE MAMMAPRINT + BLUEPRINT TEST SUITE
Analyze over a hundred relevant genes at once.
With the additional results of BluePrint, our molecular subtyping test, physicians gain an even broader genomic profile. From just one tumor block, you can interrogate over 150 genes containing information on a tumor’s level of aggression and potential for growth. These insights will serve as an essential resource for guiding your pre- and post-operative treatment strategies.