PUBLICATION: SABCS 2020
AUTHORS:
Pat Whitworth, James V Pellicane, Jr., Paul Baron, Peter Beitsch, Laura Lee, Michael Rotkis, Angela Mislowsky, Carrie Dul, Charles Nash, BichlienNguyen, Mary Murray, Paul Richards, Mark Gittleman, Stephanie Akbari, ShiyuWang, Erin B Yoder, Andrea Menicucci, Lisa Blumencranz, William Audeh, NBRST Investigators Group
Background:
MammaPrint (MP) identifies breast cancer (BC) patients who can safely forego adjuvant chemotherapy1. MP combined with the BluePrint (BP) molecular subtyping signature identifies BC subtypes with distinct therapeutic response rates and survival outcomes. In the Neoadjuvant Breast Symphony Trial (NBRST), MP and BP reclassified 22% of tumors into a different molecular subtype compared to clinical IHC/FISH methods. Furthermore, MP and BP accurately predicted rates of pathologic complete response (pCR) to neoadjuvant chemotherapy (NCT) and partial response to neoadjuvant endocrine therapy (NET)2. Here, we report 5-year overall survival (OS) and distant metastasis-free survival (DMFS) in patients from the NBRST registry according to MP and BP molecular classification
Methods:
The NBRST trial (NCT01479101) prospectively enrolled 1072 patients from 2011 to 2014, who received MP and BP testing. Patients were assigned to receive NCT or NET according to NCCN guidelines and consented to 5 years post-surgery follow-up (FU). Tumors classified by MP as High Risk (HR) or Low Risk (LR) were further stratified into four molecular subtypes by BP: Luminal-A, Luminal-B, HER2, and Basal3,4. Clinical outcomes were available for 918 patients from 67 US institutions. Median FU for OS and DMFS was 5.2 and 5.0 years, respectively. Differences in OS and DMFS at 5 years were assessed by Kaplan Meier analysis and logrank test. Clinicopathological risk assessment was performed using MINDACT criteria for clinical guidelines to classify NET patients as either clinical low risk or clinical high risk.
Results
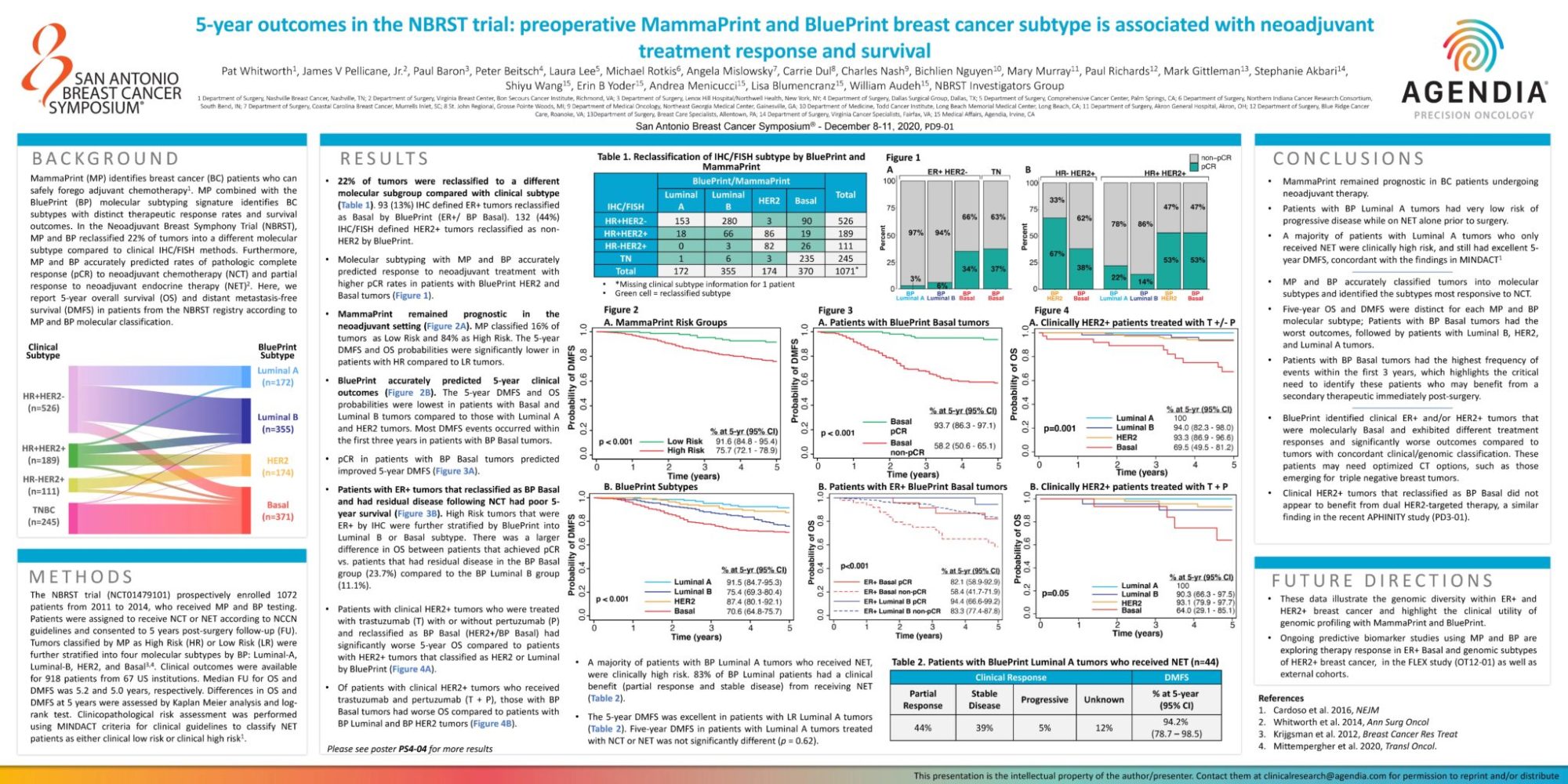
- 22% of tumors were reclassified to a different molecular subgroup compared with clinical subtype (Table 1). 93 (13%) IHC defined ER+ tumors reclassified as Basal by BluePrint (ER+/ BP Basal). 132 (44%) IHC/FISH defined HER2+ tumors reclassified as nonHER2 by BluePrint.
- Molecular subtyping with MP and BP accurately predicted response to neoadjuvant treatment with higher pCR rates in patients with BluePrint HER2 and Basal tumors (Figure 1).
- MammaPrint remained prognostic in the neoadjuvant setting (Figure 2A). MP classified 16% of tumors as Low Risk and 84% as High Risk. The 5-year DMFS and OS probabilities were significantly lower in patients with HR compared to LR tumors.
- BluePrint accurately predicted 5-year clinical outcomes (Figure 2B). The 5-year DMFS and OS probabilities were lowest in patients with Basal and Luminal B tumors compared to those with Luminal A and HER2 tumors. Most DMFS events occurred within the first three years in patients with BP Basal tumors.
- pCR in patients with BP Basal tumors predicted improved 5-year DMFS (Figure 3A).
- Patients with ER+ tumors that reclassified as BP Basal and had residual disease following NCT had poor 5-year survival (Figure 3B). High Risk tumors that were ER+ by IHC were further stratified by BluePrint into Luminal B or Basal subtype. There was a larger difference in OS between patients that achieved pCR vs. patients that had residual disease in the BP Basal group (23.7%) compared to the BP Luminal B group (11.1%).
- Patients with clinical HER2+ tumors who were treated with trastuzumab (T) with or without pertuzumab (P) and reclassified as BP Basal (HER2+/BP Basal) had significantly worse 5-year OS compared to patients with HER2+ tumors that classified as HER2 or Luminal by BluePrint (Figure 4A).
- Of patients with clinical HER2+ tumors who received trastuzumab and pertuzumab (T + P), those with BP Basal tumors had worse OS compared to patients with BP Luminal and BP HER2 tumors (Figure 4B).
- A majority of patients with BP Luminal A tumors who received NET, were clinically high risk. 83% of BP Luminal patients had a clinical benefit (partial response and stable disease) from receiving NET (Table 2).
- The 5-year DMFS was excellent in patients with LR Luminal A tumors (Table 2). Five-year DMFS in patients with Luminal A tumors treated with NCT or NET was not significantly different (p = 0.62).
Conclusion
- MammaPrint remained prognostic in BC patients undergoing neoadjuvant therapy.
- Patients with BP Luminal A tumors had very low risk of progressive disease while on NET alone prior to surgery.
- A majority of patients with Luminal A tumors who only received NET were clinically high risk, and still had excellent 5- year DMFS, concordant with the findings in MINDACT1
- MP and BP accurately classified tumors into molecular subtypes and identified the subtypes most responsive to NCT.
- Five-year OS and DMFS were distinct for each MP and BP molecular subtype; Patients with BP Basal tumors had the worst outcomes, followed by patients with Luminal B, HER2, and Luminal A tumors.
- Patients with BP Basal tumors had the highest frequency of events within the first 3 years, which highlights the critical need to identify these patients who may benefit from a secondary therapeutic immediately post-surgery.
- BluePrint identified clinical ER+ and/or HER2+ tumors that were molecularly Basal and exhibited different treatment responses and significantly worse outcomes compared to tumors with concordant clinical/genomic classification. These patients may need optimized CT options, such as those emerging for triple negative breast tumors.
- Clinical HER2+ tumors that reclassified as BP Basal did not appear to benefit from dual HER2-targeted therapy, a similar finding in the recent APHINITY study (PD3-01).