PUBLICATION:
SABCS 2020
AUTHORS:
Iris Noordhoek, Esther Bastiaannet, Ersan Lujinovic, Laura Esserman, Jelle Wesseling, Astrid Scholten, Carolien Schroders, Sjoerd Elias, Nienke de Glas, Judith Kroep, Johanneke Portielje, Miranda Kleijn, Gerrit-Jan Liefers
Introduction:
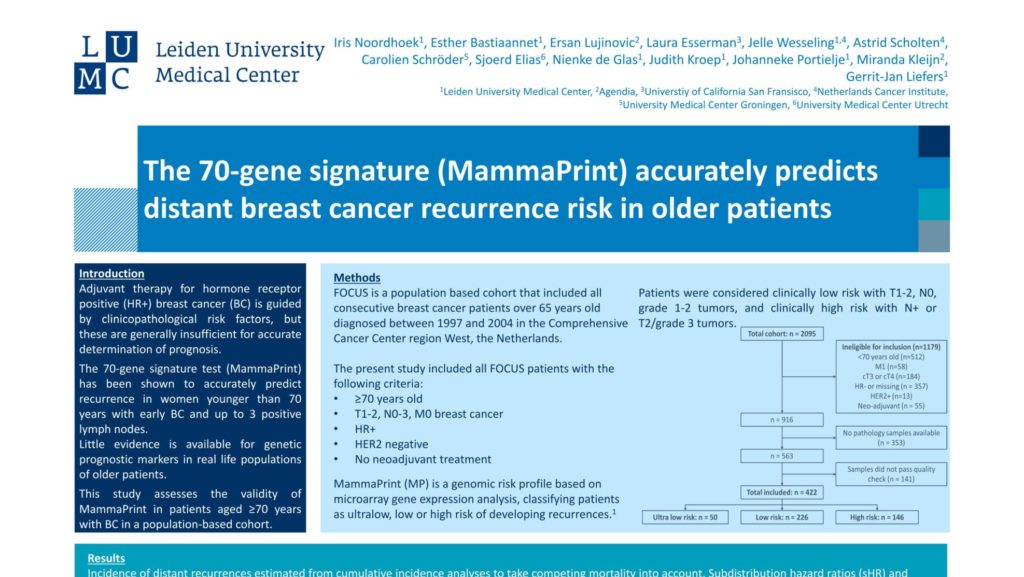
Adjuvant therapy for hormone receptor positive (HR+) breast cancer (BC) is guided by clinicopathological risk factors, but these are generally insufficient for accurate determination of prognosis. The 70-gene signature test (MammaPrint) has been shown to accurately predict recurrence in women younger than 70 years with early BC and up to 3 positive lymph nodes. Little evidence is available for genetic prognostic markers in real life populations of older patients. This study assesses the validity of MammaPrint in patients aged ≥70 years with BC in a population-based cohort.
Methods
FOCUS is a population based cohort that included all consecutive is a breast population cancer based patients cohort over that 65 years old diagnosed between region 1997 West, and the 2004 in the Comprehensive Cancer Center region West, the Netherlands.
The present study included all FOCUS patients with the following criteria:
- ≥70 years old
- Tl-2, N0-3, MO breast cancer
- HR+
- HER2 negative
- No neoadjuvant treatment
MammaPrint (MP) is a genomic risk profile based on microarray ultralow, low gene or high expression risk of analysis, developing classifying recurrences. Patients were considered clinically low risk with T1-2, N0, grade Patients 1-2 were tumors, considered and clinically low high risk with N+ or T2/grade 3 tumors.
Results
Incidence of distant recurrences estimated from cumulative 1nc1dence analyses to take competing mortality into account. Subd1stribut1on hazard ratios (sHR) and accompany 95% confidence intervals are derived from Fine and Gray analyses.
Conclusion
- MammaPrint accurately predicts 10-year distant recurrence free interval in older BC patients.
- Patients classified as ultralow risk had a very low chance of developing metastatic disease
- Even thou h 48% did not receive an ad·uvant endocrine therapy
- Clinical! hi h risk patients that received no adjuvant treatment had NO recurrent disease even 10 ears after diagnosis
- These findings are in line with published results of the STO-3 trial and may be used as the foundation for trials investigating deescalation of treatment in older patients guided by genomic testing.


