PUBLICATION: ASCO 2021
Authors
Cathy Graham1, Douglas K. Marks2, Nina D’Abreo2, Sami Diab3, Vijayakrishna K. Gadi4, Midas M. Kuilman5, Andrea Menicucci6, Amy M. Truitt6, Shiyu Wang6, Patricia Dauer6, William Audeh6, FLEX Investigators’ Group8
Background
- Recent prospective clinical trials have demonstrated a differential chemotherapy effect based on age (≤ 50 vs. > 50 years) or menopausal status (pre- vs. post-) in a genomic low risk group1,2. However, it is unclear whether these differences are a direct anti-tumor effect of chemotherapy or a secondary ovarian suppression effect caused by chemotherapy.
- In the current study, we aimed to compare the biological characteristics of breast cancer tumors from patients aged ≤ 50 years and from patients aged > 50 years using whole transcriptome analysis to provide insights into this differential chemotherapy response.
Methods
FLEX Study: The FLEX study (NCT03053193) is an ongoing, prospective study of stage I-III breast cancer patients that receive the MammaPrint (MP) 70-gene signature test with or without the BluePrint (BP) 80-gene signature test and consent to clinically annotated gene expression data collection.
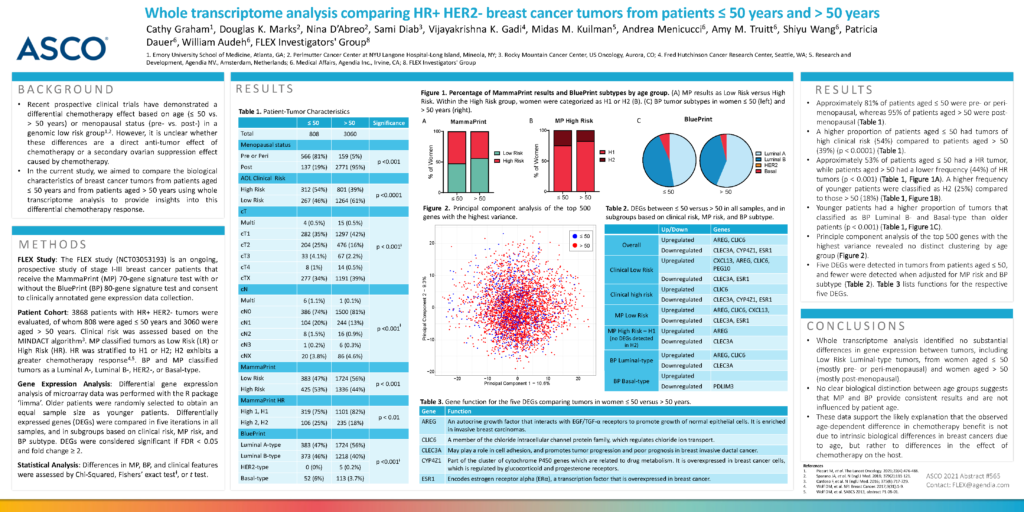
Patient Cohort: 3868 patients with HR+ HER2- tumors were evaluated, of whom 808 were aged ≤ 50 years and 3060 were aged > 50 years. Clinical risk was assessed based on the MINDACT algorithm3. MP classified tumors as Low Risk (LR) or High Risk (HR). HR was stratified to H1 or H2; H2 exhibits a greater chemotherapy response4,5. BP and MP classified tumors as a Luminal A-, Luminal B-, HER2-, or Basal-type.
Gene Expression Analysis: Differential gene expression analysis of microarray data was performed with the R package ‘limma’. Older patients were randomly selected to obtain an equal sample size as younger patients. Differentially expressed genes (DEGs) were compared in five iterations in all samples, and in subgroups based on clinical risk, MP risk, and BP subtype. DEGs were considered significant if FDR < 0.05 and fold change ≥ 2.
Statistical Analysis: Differences in MP, BP, and clinical features were assessed by Chi-Squared, Fishers’ exact testⱡ, or t test.
Results
- Approximately 81% of patients aged ≤ 50 were pre- or peri-menopausal, whereas 95% of patients aged > 50 were post-menopausal (Table 1).
- A higher proportion of patients aged ≤ 50 had tumors of high clinical risk (54%) compared to patients aged > 50 (39%) (p < 0.0001) (Table 1).
- Approximately 53% of patients aged ≤ 50 had a HR tumor, while patients aged > 50 had a lower frequency (44%) of HR tumors (p < 0.001) (Table 1, Figure 1A). A higher frequency of younger patients were classified as H2 (25%) compared to those > 50 (18%) (Table 1, Figure 1B).
- Younger patients had a higher proportion of tumors that classified as BP Luminal B- and Basal-type than older patients (p < 0.001) (Table 1, Figure 1C).
- Principle component analysis of the top 500 genes with the highest variance revealed no distinct clustering by age group (Figure 2).
- Five DEGs were detected in tumors from patients aged ≤ 50, and fewer were detected when adjusted for MP risk and BP subtype (Table 2). Table 3 lists functions for the respective five DEGs.
Conclusions
- Whole transcriptome analysis identified no substantial differences in gene expression between tumors, including Low Risk Luminal-type tumors, from women aged ≤ 50 (mostly pre- or peri-menopausal) and women aged > 50 (mostly post-menopausal).
- No clear biological distinction between age groups suggests that MP and BP provide consistent results and are not influenced by patient age.
- These data support the likely explanation that the observed age-dependent difference in chemotherapy benefit is not due to intrinsic biological differences in breast cancers due to age, but rather to differences in the effect of chemotherapy on the host.
1. Emory University School of Medicine, Atlanta, GA; 2. Perlmutter Cancer Center at NYU Langone Hospital-Long Island, Mineola, NY; 3. Rocky Mountain Cancer Center, US Oncology, Aurora, CO; 4. Fred Hutchinson Cancer Research Center, Seattle, WA; 5. Research and Development, Agendia NV., Amsterdam, Netherlands; 6. Medical Affairs, Agendia Inc., Irvine, CA; 8. FLEX Investigators’ Group