PUBLICATION:
SABCS 2020
AUTHORS:
Peter W. Blumencranz, Mehran Habibi, Lisa Blumencranz, Andrea Menicucci, Shiyu Wang, Amy Truitt, William Audeh, Jolanta L. Baginski, Steven Shivers, Geza Acs, Charles E. Cox, MINT Investigators Group.
Background:
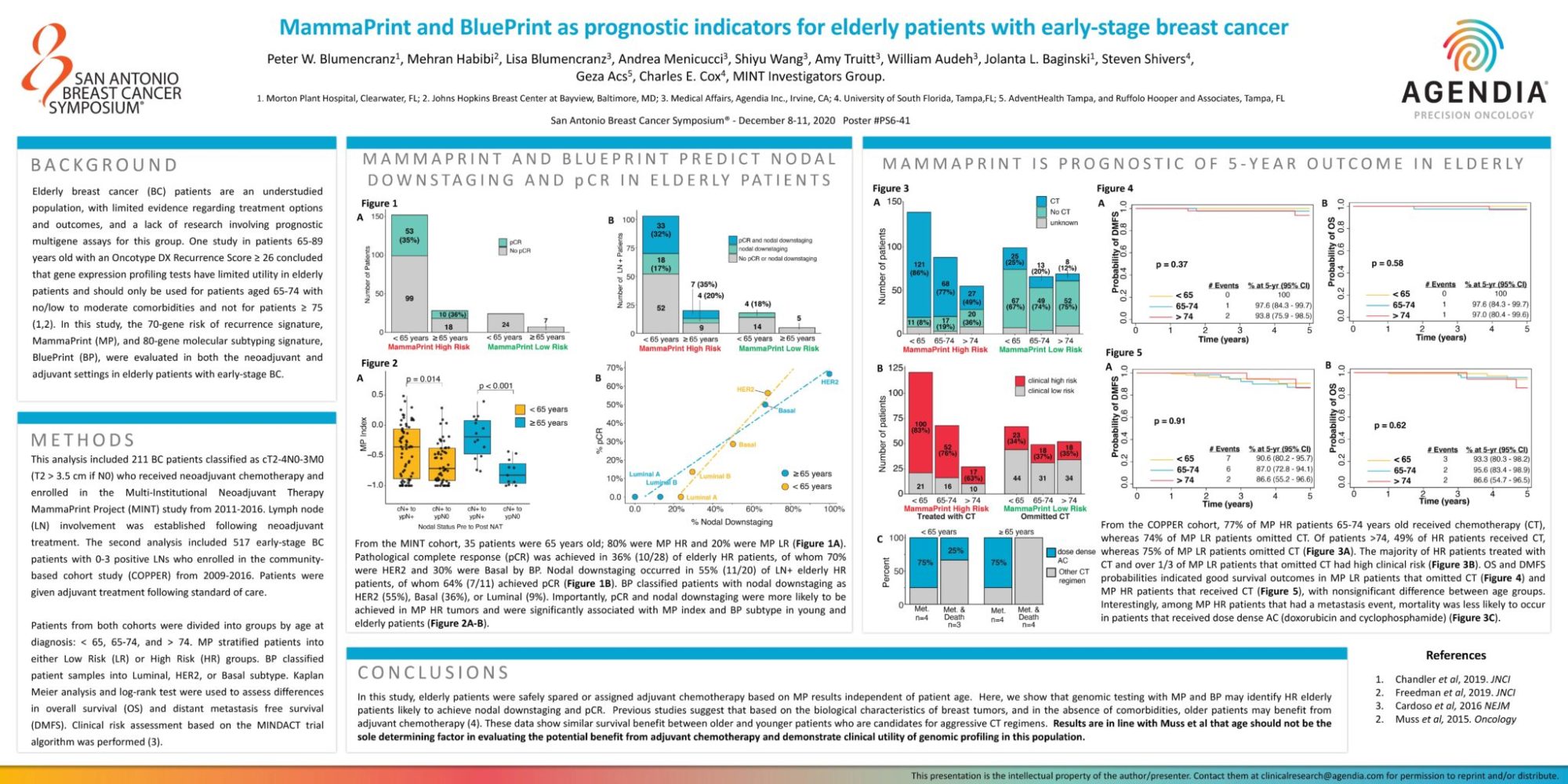
Elderly breast cancer (BC) patients are an understudied population, with limited evidence regarding treatment options and outcomes, and a lack of research involving prognostic multigene assays for this group. One study in patients 65-89 years old with an Oncotype DX Recurrence Score ≥ 26 concluded that gene expression profiling tests have limited utility in elderly patients and should only be used for patients aged 65-74 with no/low to moderate comorbidities and not for patients ≥ 75 (1,2). In this study, the 70-gene risk of recurrence signature, MammaPrint (MP), and 80-gene molecular subtyping signature, BluePrint (BP), were evaluated in both the neoadjuvant and adjuvant settings in elderly patients with early-stage BC.
Methods
This analysis included 211 BC patients classified as cT2-4N0-3M0 (T2 > 3.5 cm if N0) who received neoadjuvant chemotherapy and enrolled in the Multi-Institutional Neoadjuvant Therapy MammaPrint Project (MINT) study from 2011-2016. Lymph node (LN) involvement was established following neoadjuvant treatment. The second analysis included 517 early-stage BC patients with 0-3 positive LNs who enrolled in the community-based cohort study (COPPER) from 2009-2016. Patients were given adjuvant treatment following standard of care.
Patients from both cohorts were divided into groups by age at diagnosis: < 65, 65-74, and > 74. MP stratified patients into either Low Risk (LR) or High Risk (HR) groups. BP classified patient samples into Luminal, HER2, or Basal subtype. Kaplan Meier analysis and log-rank test were used to assess differences in overall survival (OS) and distant metastasis free survival (DMFS). Clinical risk assessment based on the MINDACT trial algorithm was performed (3).
Conclusions
In this study, elderly patients were safely spared or assigned adjuvant chemotherapy based on MP results independent of patient age. Here, we show that genomic testing with MP and BP may identify HR elderly patients likely to achieve nodal downstaging and pCR. Previous studies suggest that based on the biological characteristics of breast tumors, and in the absence of comorbidities, older patients may benefit from adjuvant chemotherapy (4). These data show similar survival benefit between older and younger patients who are candidates for aggressive CT regimens. Results are in line with Muss et al that age should not be the sole determining factor in evaluating the potential benefit from adjuvant chemotherapy and demonstrate clinical utility of genomic profiling in this population.