PUBLICATION:
SABCS 2020
AUTHORS:
Joseph McKelley, Jennifer Wei, Brian Hoxeng, Andrea Menicucci, Erin Yoder, Shiyu Wang, William Audeh
Background:
Pre-operative/neoadjuvant treatment utilization in early-stage breast cancer has been increasing, particularly during the COVID-19 pandemic. With goals of minimizing potential exposure to SARS-COV-2, as well as resource rationing, physicians are urged to triage breast cancer patients by identifying those who require urgent surgical care vs. those who may delay surgical treatment. Accurate risk assessment is an integral component of this triaging process, and recent guidelines have recommended that genomic testing on diagnostic core needle biopsy (CNB) samples be used to assist with the identification of patients with low risk tumor biology who may be candidates for surgical delay.
• MammaPrint (MP), a 70-gene risk of recurrence signature, and BluePrint (BP), an 80-gene molecular subtyping signature, have been routinely used in formalin-fixed paraffin embedded (FFPE) CNB and surgical resection (SR) samples since MammaPrint obtained FDA clearance for FFPE tissue in 20152.
• In addition, over 1,500 CNB tumor samples from patients enrolled in prospective neoadjuvant treatment trials (NBRST3 and ISPY-24) have received successful MP & BP testing. This study compares the gene expression results between CNB and SR specimens to better elucidate how these tests perform across specimen type.
Methods
Routine diagnostic samples submitted to Agendia, Inc. (Irvine, CA) between February 2017 and May 2020 for MP and BP testing were processed according to standard FFPE microarray procedures. MP was used to stratify samples into Low Risk (LR) and High Risk (HR). An additional subset analysis of the LR group was performed to identify patients with an Ultralow Risk (UL) result [MPI > +0.355]. BP was used to classify samples into Basal, Luminal or HER2-Type.
This study included 13,603 CNB and 25,684 SR specimens. The majority of samples classified as BP Luminal-Type amongst both specimen types (>85%), therefore we compared MP Index (MPI) distribution between CNB and SR samples for Luminal-Type tumors. Comparative “logistics metrics” (average turnaround time [TAT] and success rate) were also assessed between these specimen types.
Results
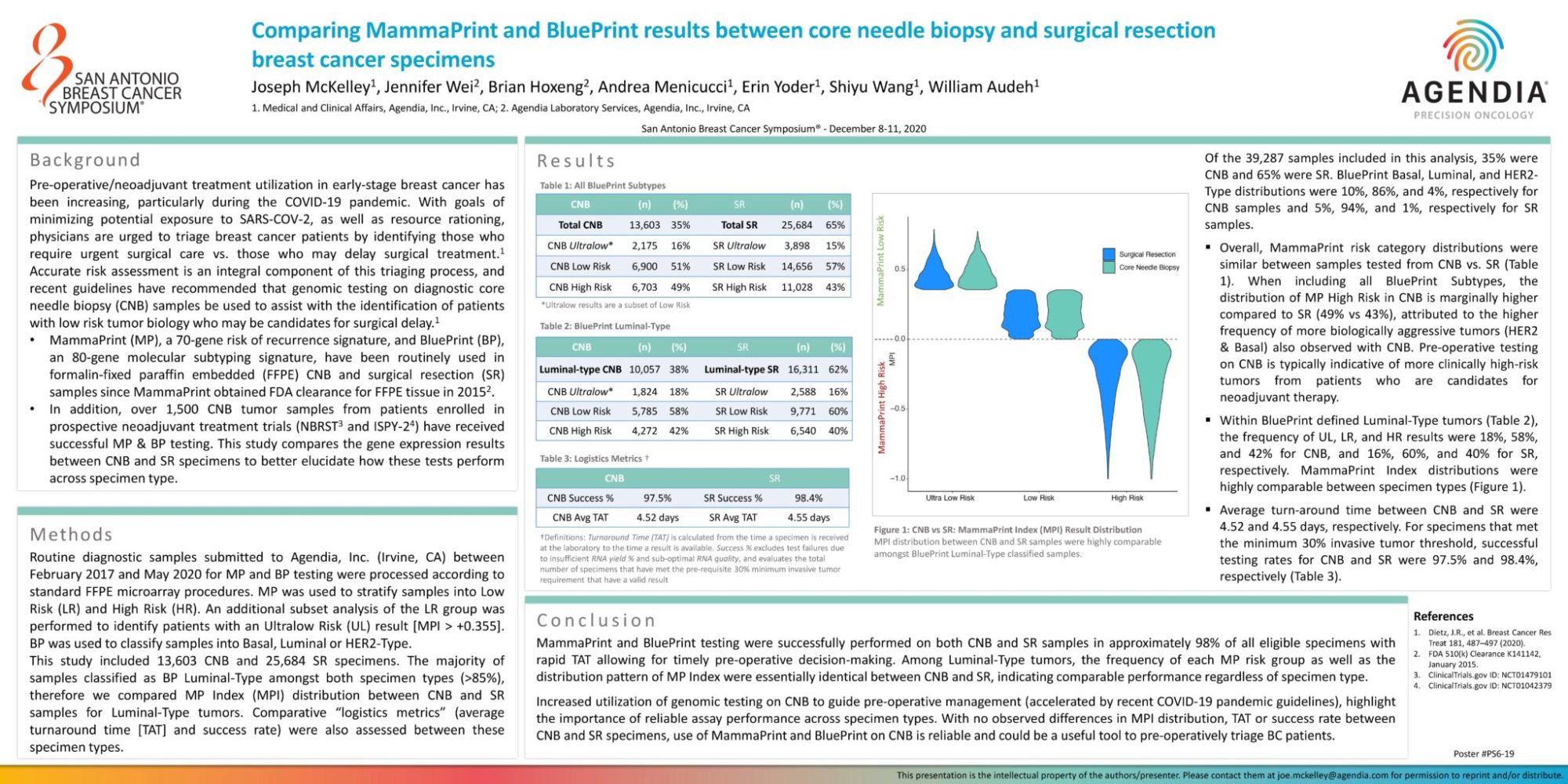
Of the 39,287 samples included in this analysis, 35% were CNB and 65% were SR. BluePrint Basal, Luminal, and HER2-Type distributions were 10%, 86%, and 4%, respectively for CNB samples and 5%, 94%, and 1%, respectively for SR samples.
- Overall, MammaPrint risk category distributions were similar between samples tested from CNB vs. SR (Table 1). When including all BluePrint Subtypes, the distribution of MP High Risk in CNB is marginally higher compared to SR (49% vs 43%), attributed to the higher frequency of more biologically aggressive tumors (HER2 & Basal) also observed with CNB. Pre-operative testing on CNB is typically indicative of more clinically high-risk tumors from patients who are candidates for neoadjuvant therapy.
- Within BluePrint defined Luminal-Type tumors (Table 2), the frequency of UL, LR, and HR results were 18%, 58%, and 42% for CNB, and 16%, 60%, and 40% for SR, respectively. MammaPrint Index distributions were highly comparable between specimen types (Figure 1).
- Average turn-around time between CNB and SR were 4.52 and 4.55 days, respectively. For specimens that met the minimum 30% invasive tumor threshold, successful testing rates for CNB and SR were 97.5% and 98.4%, respectively (Table 3).
Conclusions
MammaPrint and BluePrint testing were successfully performed on both CNB and SR samples in approximately 98% of all eligible specimens with rapid TAT allowing for timely pre-operative decision-making. Among Luminal-Type tumors, the frequency of each MP risk group as well as the distribution pattern of MP Index were essentially identical between CNB and SR, indicating comparable performance regardless of specimen type.
Increased utilization of genomic testing on CNB to guide pre-operative management (accelerated by recent COVID-19 pandemic guidelines), highlight the importance of reliable assay performance across specimen types. With no observed differences in MPI distribution, TAT or success rate between CNB and SR specimens, use of MammaPrint and BluePrint on CNB is reliable and could be a useful tool to pre-operatively triage BC patients.