PUBLICATION:
SABCS 2020
AUTHORS:
Pat Whitworth, James V Pellicane, Jr., Paul Baron, Peter Beitsch, Laura Lee, Michael Rotkis, Angela Mislowsky, Carrie Dul, Charles Nash, Bichlien Nguyen, Mary Murray, Paul Richards, Mark Gittleman13, Stephanie Akbari, Shiyu Wang, Andrea Menicucci, Erin B Yoder, Lisa Blumencranz, William Audeh, NBRST Investigators Group
Background:
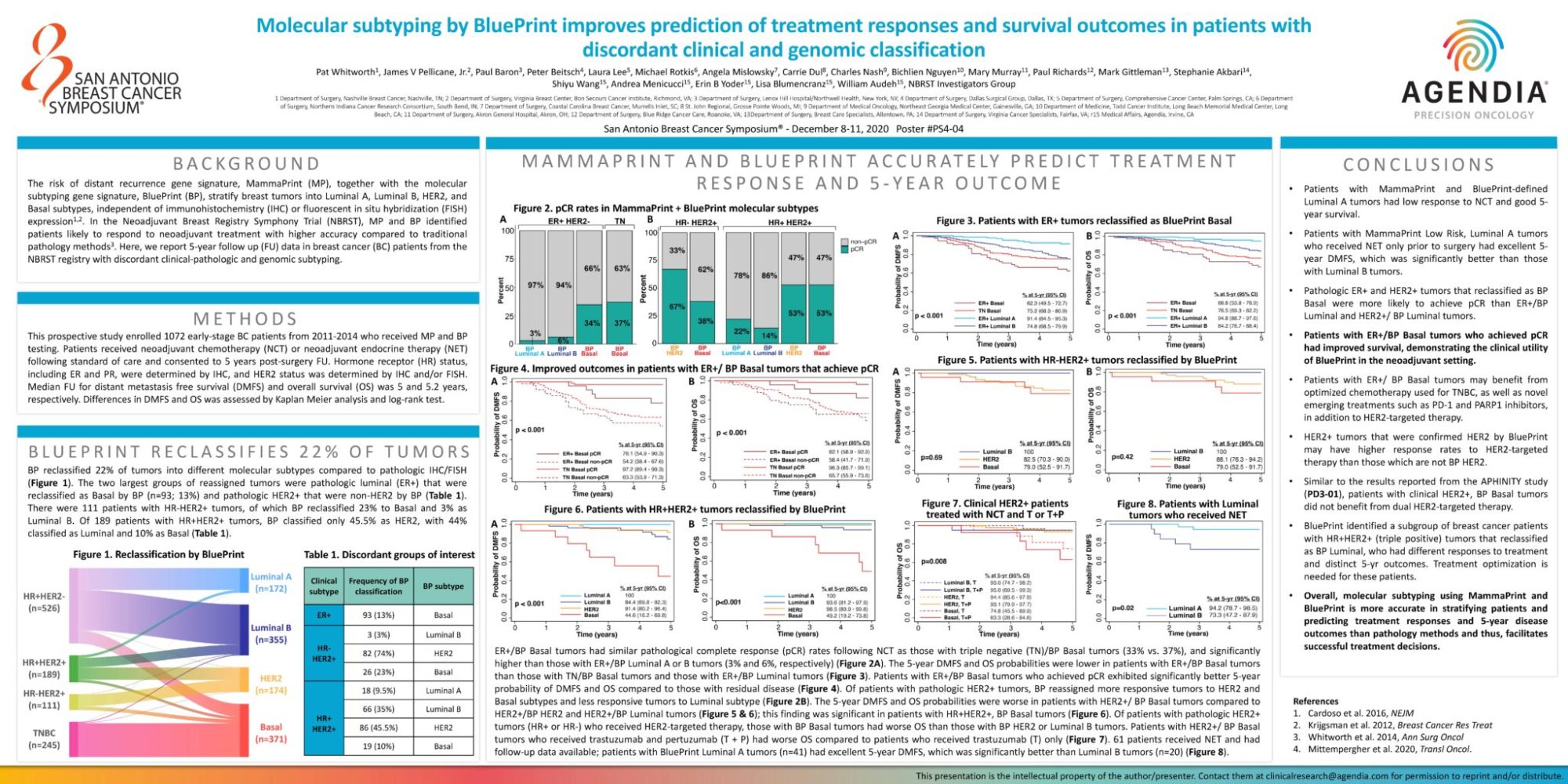
The risk of distant recurrence gene signature, MammaPrint (MP), together with the molecular subtyping gene signature, BluePrint (BP), stratify breast tumors into Luminal A, Luminal B, HER2, and Basal subtypes, independent of immunohistochemistry (IHC) or fluorescent in situ hybridization (FISH) expression1,2. In the Neoadjuvant Breast Registry Symphony Trial (NBRST), MP and BP identified patients likely to respond to neoadjuvant treatment with higher accuracy compared to traditional pathology methods3. Here, we report 5-year follow up (FU) data in breast cancer (BC) patients from the NBRST registry with discordant clinical-pathologic and genomic subtyping.
Methods
This prospective study enrolled 1072 early-stage BC patients from 2011-2014 who received MP and BP testing. Patients received neoadjuvant chemotherapy (NCT) or neoadjuvant endocrine therapy (NET) following standard of care and consented to 5 years post-surgery FU. Hormone receptor (HR) status, including ER and PR, were determined by IHC, and HER2 status was determined by IHC and/or FISH. Median FU for distant metastasis free survival (DMFS) and overall survival (OS) was 5 and 5.2 years, respectively. Differences in DMFS and OS was assessed by Kaplan Meier analysis and log-rank test.
BluePrint Reclassifies 22% of Tumors
BP reclassified 22% of tumors into different molecular subtypes compared to pathologic IHC/FISH (Figure 1). The two largest groups of reassigned tumors were pathologic luminal (ER+) that were reclassified as Basal by BP (n=93; 13%) and pathologic HER2+ that were non-HER2 by BP (Table 1). There were 111 patients with HR-HER2+ tumors, of which BP reclassified 23% to Basal and 3% as Luminal B. Of 189 patients with HR+HER2+ tumors, BP classified only 45.5% as HER2, with 44%classified as Luminal and 10% as Basal (Table 1).
MammaPrint and BluePrint Accurately predict treatment response and 5-year outcome
ER+/BP Basal tumors had similar pathological complete response (pCR) rates following NCT as those with triple negative (TN)/BP Basal tumors (33% vs. 37%), and significantly higher than those with ER+/BP Luminal A or B tumors (3% and 6%, respectively) (Figure 2A). The 5-year DMFS and OS probabilities were lower in patients with ER+/BP Basal tumors than those with TN/BP Basal tumors and those with ER+/BP Luminal tumors (Figure 3). Patients with ER+/BP Basal tumors who achieved pCR exhibited significantly better 5-year probability of DMFS and OS compared to those with residual disease (Figure 4). Of patients with pathologic HER2+ tumors, BP reassigned more responsive tumors to HER2 and Basal subtypes and less responsive tumors to Luminal subtype (Figure 2B). The 5-year DMFS and OS probabilities were worse in patients with HER2+/ BP Basal tumors compared to HER2+/BP HER2 and HER2+/BP Luminal tumors (Figure 5 & 6); this finding was significant in patients with HR+HER2+, BP Basal tumors (Figure 6). Of patients with pathologic HER2+ tumors (HR+ or HR-) who received HER2-targeted therapy, those with BP Basal tumors had worse OS than those with BP HER2 or Luminal B tumors. Patients with HER2+/ BP Basal tumors who received trastuzumab and pertuzumab (T + P) had worse OS compared to patients who received trastuzumab (T) only (Figure 7). 61 patients received NET and had follow-up data available; patients with BluePrint Luminal A tumors (n=41) had excellent 5-year DMFS, which was significantly better than Luminal B tumors (n=20) (Figure 8).
Conclusions
• Patients with MammaPrint and BluePrint-defined Luminal A tumors had low response to NCT and good 5-year survival.
• Patients with MammaPrint Low Risk, Luminal A tumors who received NET only prior to surgery had excellent 5-year DMFS, which was significantly better than those with Luminal B tumors.
• Pathologic ER+ and HER2+ tumors that reclassified as BP Basal were more likely to achieve pCR than ER+/BP Luminal and HER2+/ BP Luminal tumors.
• Patients with ER+/BP Basal tumors who achieved pCR had improved survival, demonstrating the clinical utility of BluePrint in the neoadjuvant setting.
• Patients with ER+/ BP Basal tumors may benefit from optimized chemotherapy used for TNBC, as well as novel emerging treatments such as PD-1 and PARP1 inhibitors, in addition to HER2-targeted therapy.
• HER2+ tumors that were confirmed HER2 by BluePrint may have higher response rates to HER2-targeted therapy than those which are not BP HER2.
• Similar to the results reported from the APHINITY study (PD3-01), patients with clinical HER2+, BP Basal tumors did not benefit from dual HER2-targeted therapy.
• BluePrint identified a subgroup of breast cancer patients with HR+HER2+ (triple positive) tumors that reclassified as BP Luminal, who had different responses to treatment and distinct 5-yr outcomes. Treatment optimization is needed for these patients.
• Overall, molecular subtyping using MammaPrint and BluePrint is more accurate in stratifying patients and predicting treatment responses and 5-year disease outcomes than pathology methods and thus, facilitates successful treatment decisions.