Title: Utility of the MammaPrint 70-Gene and BluePrint 80-Gene Expression Signatures in Providing Personalized Treatment Decision in Early-Stage Breast Cancer, and the Role of the Oncology Nurse Navigator: A Case-Study Approach
Publication: Journal of Oncology Navigation & Survivorship, November 2023, Vol 14
Authors
Jo Weathers, RN, BSN, OCN, ONN-CG; Jane Baker, RN, MSN; Victoria Poillucci, DNP, MEd, ACNP-BC; Christina Z. Page, AOCNP, AGPCNP-BC
Background
Nurse navigators (NNs) are key players on the oncology care team who educate their patients and advocate for them, while facilitating comprehensive care. MammaPrint is a 70-gene test that assesses the risk of distant metastasis in early-stage breast cancer. BluePrint is an 80-gene test that identifies the functional molecular pathways that drive tumor growth in an individual breast cancer. Together, the results of these tests provide valuable information that guides treatment decisions.
Objective
Through a case-study approach, we aim to demonstrate how oncology NNs can utilize the gene expression signatures of breast cancers, obtained by MammaPrint and BluePrint assays, to guide their patients through treatment-related decision-making at multiple points throughout their cancer journey.
Methods
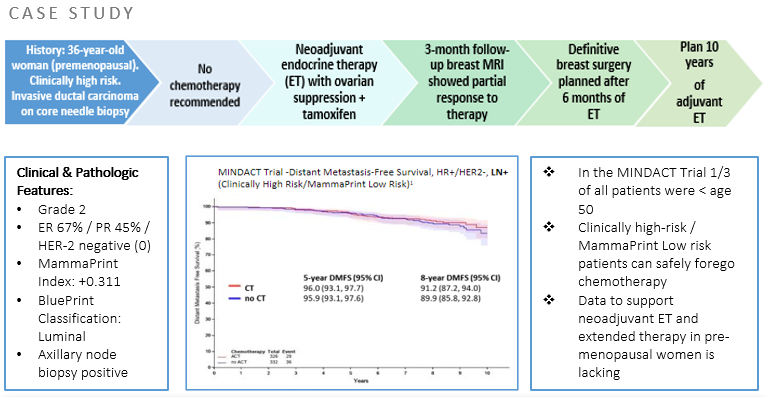
An oncology NN from a community-based clinic in the Southeast identified a case study in which the results of the MammaPrint and BluePrint genomic assays were important in determining the treatment plan. The NN advocated for and facilitated the order of the MammaPrint and BluePrint tests from the core needle biopsy. The results of these tests allowed a breadth of information about this patient’s tumor to be available at the time of the initial medical oncology visit. Clinical risk was determined by evaluating the tumor size, grade, and the status of estrogen receptor (ER) and progesterone receptor (PR), human epidermal growth factor receptor-2 (HER2), and lymph node spread. MammaPrint, the 70-gene signature, categorizes tumor risk of recurrence as UltraLow (+0.356 to +1.000), Low (+0.355 to >0.000), High1 (0.000 to -0.569) and High2 (-0.570 to -1.000). BluePrint, the 80-gene signature, further classifies tumors as Luminal, HER2 type, or Basal. These classifications most accurately predict sensitivity to chemotherapy and endocrine therapy, pathologic complete response rates, and long-term outcomes.
Results
A 36-year-old premenopausal woman with a clinically high-risk tumor had a core biopsy of the right breast revealing grade 2 invasive ductal carcinoma. Biomarkers showed ER 67%, PR 45%, and HER2 negative (0). A MammaPrint Index of +0.311 classified the tumor as Low Risk, and BluePrint further classified the tumor as Luminal A. A right axillary lymph node biopsy was positive. The patient was recommended neoadjuvant endocrine therapy consisting of ovarian function suppression with leuprolide and daily antiestrogen with tamoxifen. After 3 months of therapy, repeat breast imaging showed a partial response to therapy. MammaPrint/BluePrint Low-risk Luminal results indicate endocrine sensitivity. Endocrine therapy is ongoing. Surgery is planned after 6 months of therapy. Ten years of adjuvant endocrine therapy is recommended. Chemotherapy is not recommended.
Data to inform the care of young women with breast cancer are lacking. Genomic information supplemented clinicopathologic information and facilitated shared decision-making involving the NN in this challenging case.
Conclusions
When considering clinicopathologic features alone, patients with clinically high-risk tumors are often recommended chemotherapy. The initial recommendation for neoadjuvant chemotherapy was adjusted after review of MammaPrint and BluePrint results, suggesting that this patient could safely forego chemotherapy and receive endocrine therapy alone. As key figures in a patient’s cancer journey, NNs are poised to advocate for the use of decision-making tools that can further personalize care. The MammaPrint and BluePrint genomic assays increase the confidence in treatment decisions for patients and providers. Continuous education of NNs on genomic assays remains important to facilitate shared decision-making and improve timely care.


