Publication: ESMO BREAST CANCER CONGRESS 2022
Authors
Esther Schuler(1), Sahra Uygun(2), Lorenza Mittempergher(3), Darina Pronin(3), Sammy Mee(2), Simon Bao(2), Tyson Cavness(2), Anke Witteveen(3), and Annuska Glas(3)
1. Zotz|Klimas, MVZ Düsseldorf-Centrum GbR, Germany 2. Agendia Inc., Irvine, CA 3. Agendia NV., Amsterdam, Netherlands
Background
The MammaPrint® 70-gene signature (70GS) risk of recurrence and BluePrint® 80-gene signature (80GS) of molecular subtyping are utilized in personalized treatment planning of early-stage breast cancer patients. These gene expression assays were developed with microarray (MA) and have been translated into Next Generation Sequencing (NGS) using targeted RNA-sequencing and CE marked. Equivalence between MA and NGS has been shown with NGS in centralized and decentralized setting1-3. Here we present extended data from multiple global sites and report performance on equivalence.
Methods
A total of 1,192 paired results from MA and NGS test corresponding to 993 unique samples were used in this study. All MA results were generated in Agendia’s central laboratory. A total of 689 NGS results were generated at Agendia, and 503 NGS results were generated across 11 external partner sites in 5 different countries. Comparisons of the test indices and result categories were conducted. Reproducibility was assessed with standard deviation from the samples that have repeated NGS measurements. Additionally, NGS testing from two RNA isolations of the same tissue was performed and concordance was evaluated among a subset of samples to assess the impact on 70GS results. 70GS risk categories include Low Risk and High Risk. 80GS molecular subtypes include Basal-, Luminal-, and HER2-type.
Results
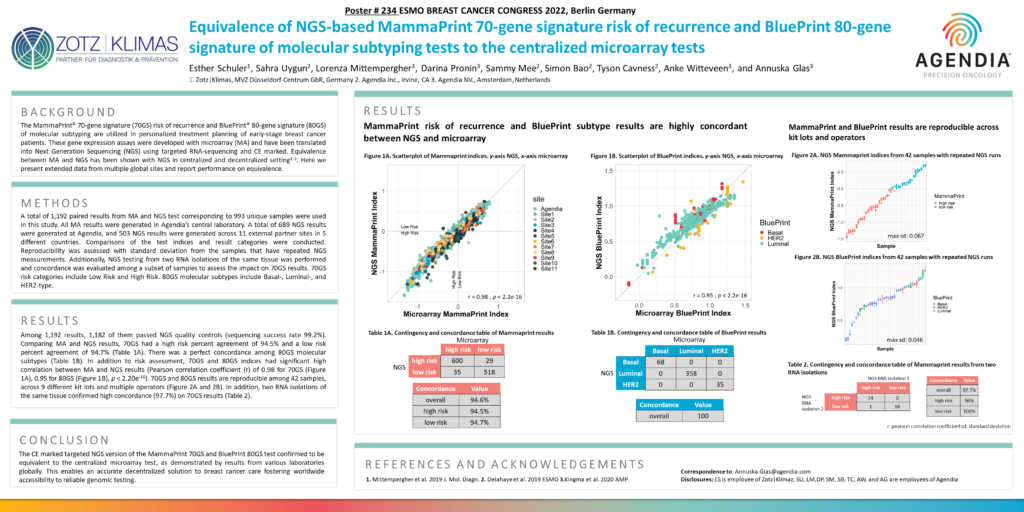
Among 1,192 results, 1,182 of them passed NGS quality controls (sequencing success rate 99.2%). Comparing MA and NGS results, 70GS had a high risk percent agreement of 94.5% and a low risk percent agreement of 94.7% (Table 1A). There was a perfect concordance among 80GS molecular subtypes (Table 1B). In addition to risk assessment, 70GS and 80GS indices had significant high correlation between MA and NGS results (Pearson correlation coefficient (r) of 0.98 for 70GS (Figure 1A), 0.95 for 80GS (Figure 1B), p < 2.20e-16). 70GS and 80GS results are reproducible among 42 samples, across 9 different kit lots and multiple operators (Figure 2A and 2B). In addition, two RNA isolations of the same tissue confirmed high concordance (97.7%) on 70GS results (Table 2).
Conclusion
The CE marked targeted NGS version of the MammaPrint 70GS and BluePrint 80GS test confirmed to be equivalent to the centralized microarray test, as demonstrated by results from various laboratories globally. This enables an accurate decentralized solution to breast cancer care fostering worldwide accessibility to reliable genomic testing.
References and Acknowledgments
- Mittempergher et al. 2019 J. Mol. Diagn. 2. Delahaye et al. 2019 ESMO 3.Kingma et al. 2020 AMP


