Source: San Antonio Breast Cancer Symposium®
Authors: Robert Maganini, Ellis Levine, Kent Hoskins, Sarah Thayer, Alfredo Santillan, Sung HoLee, Eduardo Dias, Regina Hampton, Eric Brow
Title: FLEX: A Real-World Evidence, Full Transcriptome Study in 30,000 Patients With Early-Stage Breast Cancer
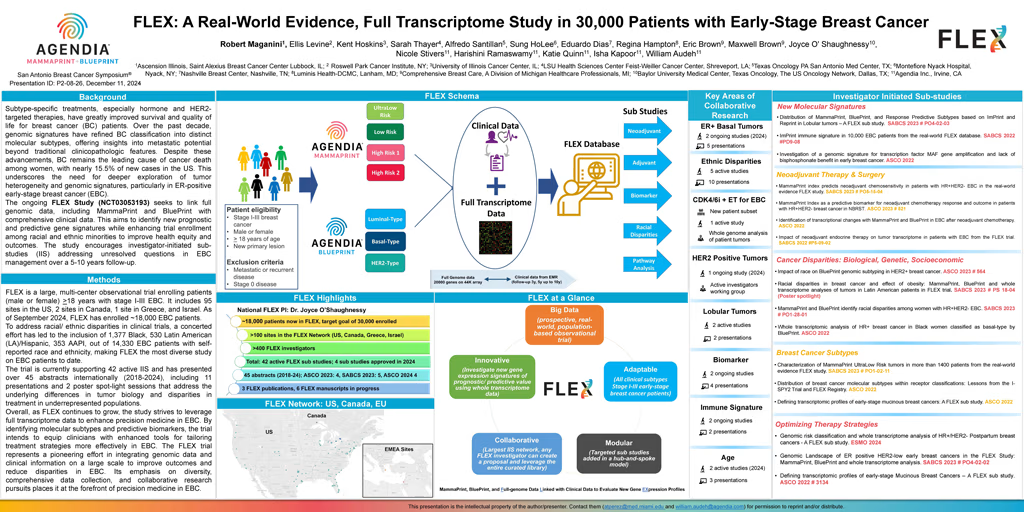
Background: The advent of subtype-specific treatments, particularly hormone therapies and HER2-targeted therapies, has significantly improved survival rates and quality of life for breast cancer (BC) patients. Over the last decade genomic signatures have enabled improved classification of BC into distinct molecular subtypes, providing prognostic and/or predictive information about the metastatic potential of the tumor beyond those of clinicopathologic features. Despite marked progress, BC remains the most frequent cause of cancer death among women globally, accounting for almost 15.5% of all new female BC cases in the US. These poor clinical outcomes warrant further understanding of tumor heterogeneity and identifying genomic signatures, particularly variation within the subset of ER positive early-stage breast cancer (EBC). Pairing the full genome expression data with comprehensive clinical information enables further tumor stratification and a deeper understanding of tumor biology driving EBC.
The ongoing, multi-center FLEX study (NCT03053193) seeks to enroll 30,000 patients to create a large-scale, diverse, population-based registry of full genome expression data matched with clinical data to investigate new gene expression signatures of prognostic and/or predictive value in a real-world setting. Efforts are focused on increasing clinical trial enrollment of racial/ethnic minorities and other historically underrepresented groups in clinical trials in the US to promote efficacy in outcomes and health equity. Additional objectives include supporting investigator-initiated sub-studies to address yet unresolved clinically relevant questions in EBC over 5-10 years of follow-up.
Methods: FLEX is a large, multi-center, prospective, observational trial that enrolls patients (male or female) who are ≥ 18 years old with histologically proven stage I-III breast cancer. All patients with up to 3 positive lymph nodes who receive standard of care MammaPrint (70-gene signature risk of recurrence), with or without BluePrint (80-gene signature molecular subtype) on a primary breast tumor and consent to clinically annotated full transcriptome data collection are eligible for enrollment. FLEX fosters collaboration across 95 sites in the US, 2 sites in Canada, 1 site in Greece, and Israel. This initiative encourages investigator-initiated sub-studies, promoting diverse research perspectives and potentially enhancing the scope and robustness of the overall study. Within 7 years of trial initiation, FLEX total enrollment amounts to 16,980 EBC patients. To address racial/ ethnic disparities in clinical trials, a concerted effort has led to the inclusion of 1,377 Black, 530 Latin American (LA)/Hispanic, 353 AAPI, out of 14,330 EBC patients with self-reported race and ethnicity, making FLEX the most diverse study on EBC patients to date. Such diversity in FLEX sets a valuable precedent for future research aiming to improve healthcare outcomes for all groups. Currently FLEX supports 12 in-progress investigator initiated sub-studies in 2024, with over 45 abstracts accepted at congresses internationally (2018-2024), including 11 presentations and 2 poster spot-light sessions that address the underlying differences in tumor biology and clinical outcomes in Black, LA, and AAPI populations.
Overall, as FLEX continues to grow, the study strives to leverage full transcriptome data to enhance precision medicine in EBC. By identifying molecular subtypes and predictive biomarkers, the trial intends to equip clinicians with enhanced tools for tailoring treatment strategies more effectively in EBC. The FLEX trial represents a pioneering effort in integrating genomic data and clinical information on a large scale to improve outcomes and reduce disparities in EBC. Its emphasis on diversity, comprehensive data collection, and collaborative research pursuits places it at the forefront of precision medicine in EBC.