PUBLICATION:
SABCS 2020
AUTHORS:
Virginia G. Kaklamani, Cathy Graham, Karen L. Tedesco, Abirami Sivapiragasam, Jennifer A. Crozier, Apurva N. Shah, Yuan Yuan, Josien Haan,
Andrea Menicucci, Michelle L. Bolner, Shiyu Wang, Lorenza Mittempergher, Erin Yoder, William Audeh9, FLEX Investigators’ Group
Background:
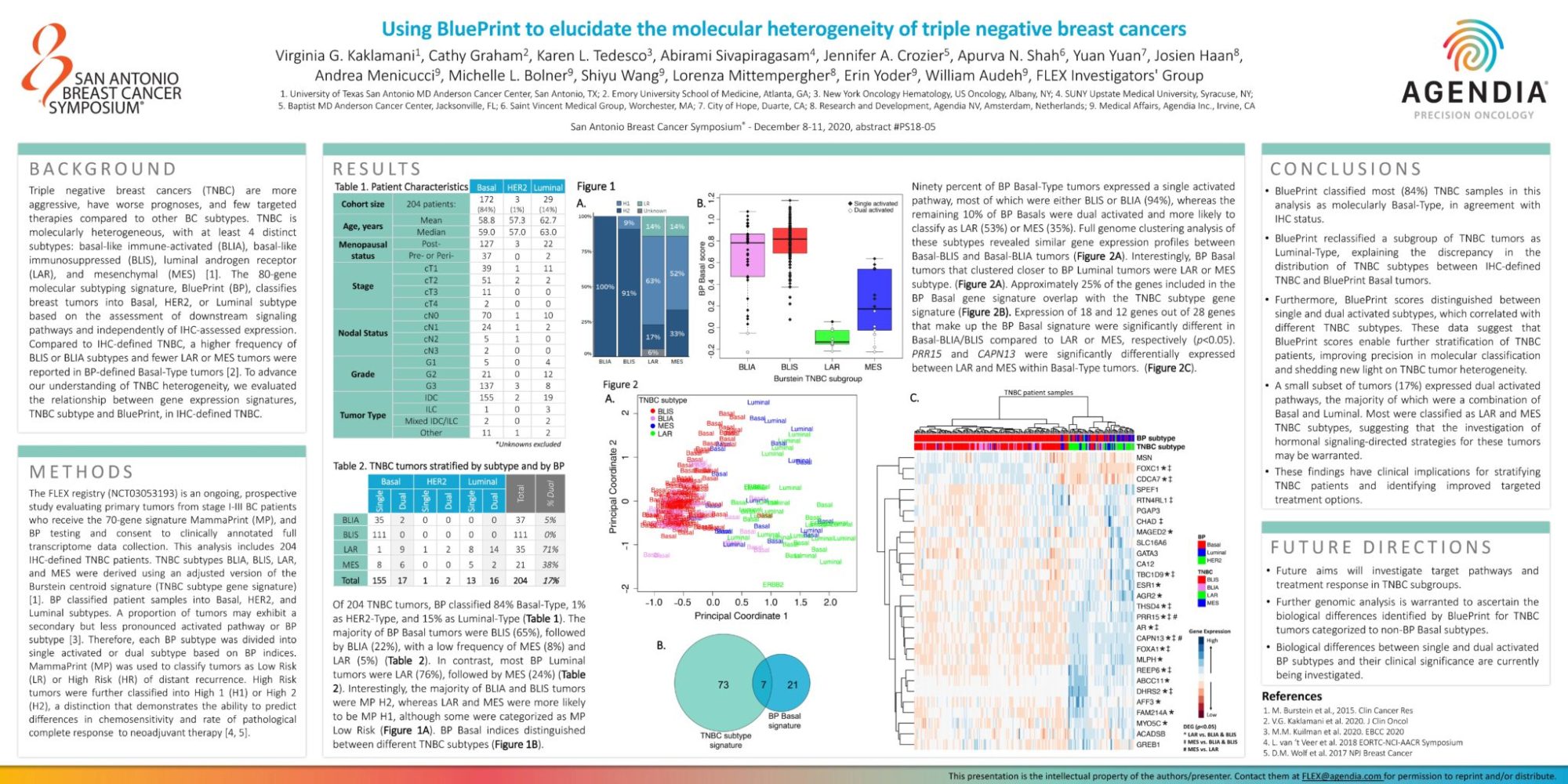
Triple negative breast cancers (TNBC) are more aggressive, have worse prognoses, and few targeted therapies compared to other BC subtypes. TNBC is molecularly heterogeneous, with at least 4 distinct subtypes: basal-like immune-activated (BLIA), basal-like immunosuppressed (BLIS), luminal androgen receptor (LAR), and mesenchymal (MES) [1]. The 80-gene molecular subtyping signature, BluePrint (BP), classifies breast tumors into Basal, HER2, or Luminal subtype based on the assessment of downstream signaling pathways and independently of IHC-assessed expression. Compared to IHC-defined TNBC, a higher frequency of BLIS or BLIA subtypes and fewer LAR or MES tumors were reported in BP-defined Basal-Type tumors [2]. To advance our understanding of TNBC heterogeneity, we evaluated the relationship between gene expression signatures, TNBC subtype and BluePrint, in IHC-defined TNBC.
Methods
The FLEX registry (NCT03053193) is an ongoing, prospective study evaluating primary tumors from stage I-III BC patients who receive the 70-gene signature MammaPrint (MP), and BP testing and consent to clinically annotated full transcriptome data collection. This analysis includes 204 IHC-defined TNBC patients. TNBC subtypes BLIA, BLIS, LAR, and MES were derived using an adjusted version of the Burstein centroid signature (TNBC subtype gene signature)[1]. BP classified patient samples into Basal, HER2, and Luminal subtypes. A proportion of tumors may exhibit a secondary but less pronounced activated pathway or BP subtype [3]. Therefore, each BP subtype was divided into single activated or dual subtype based on BP indices. MammaPrint (MP) was used to classify tumors as Low Risk (LR) or High Risk (HR) of distant recurrence. High Risk tumors were further classified into High 1 (H1) or High 2 (H2), a distinction that demonstrates the ability to predict differences in chemosensitivity and rate of pathological complete response to neoadjuvant therapy [4, 5].
Results
Of 204 TNBC tumors, BP classified 84% Basal-Type, 1%as HER2-Type, and 15% as Luminal-Type (Table 1). The majority of BP Basal tumors were BLIS (65%), followed by BLIA (22%), with a low frequency of MES (8%) and LAR (5%) (Table 2). In contrast, most BP Luminal tumors were LAR (76%), followed by MES (24%) (Table 2). Interestingly, the majority of BLIA and BLIS tumors were MP H2, whereas LAR and MES were more likely to be MP H1, although some were categorized as MP Low Risk (Figure 1A). BP Basal indices distinguished between different TNBC subtypes (Figure 1B).
Ninety percent of BP Basal-Type tumors expressed a single activated pathway, most of which were either BLIS or BLIA (94%), whereas the remaining 10% of BP Basals were dual activated and more likely to classify as LAR (53%) or MES (35%). Full genome clustering analysis of these subtypes revealed similar gene expression profiles between Basal-BLIS and Basal-BLIA tumors (Figure 2A). Interestingly, BP Basal tumors that clustered closer to BP Luminal tumors were LAR or MES subtype. (Figure 2A). Approximately 25% of the genes included in the BP Basal gene signature overlap with the TNBC subtype gene signature (Figure 2B). Expression of 18 and 12 genes out of 28 genes that make up the BP Basal signature were significantly different in Basal-BLIA/BLIS compared to LAR or MES, respectively (p<0.05). PRR15 and CAPN13 were significantly differentially expressed between LAR and MES within Basal-Type tumors. (Figure 2C).
Conclusion
• BluePrint classified most (84%) TNBC samples in this analysis as molecularly Basal-Type, in agreement with IHC status.
• BluePrint reclassified a subgroup of TNBC tumors as Luminal-Type, explaining the discrepancy in the distribution of TNBC subtypes between IHC-defined TNBC and BluePrint Basal tumors.
• Furthermore, BluePrint scores distinguished between single and dual activated subtypes, which correlated with different TNBC subtypes. These data suggest that BluePrint scores enable further stratification of TNBC patients, improving precision in molecular classification and shedding new light on TNBC tumor heterogeneity.
• A small subset of tumors (17%) expressed dual activated pathways, the majority of which were a combination of Basal and Luminal. Most were classified as LAR and MES TNBC subtypes, suggesting that the investigation of hormonal signaling-directed strategies for these tumors may be warranted.
• These findings have clinical implications for stratifying TNBC patients and identifying improved targeted treatment options.