Publication: Miami Breast
Authors:
Pat Whitworth, Jennifer A. Crozier, Robert Maganini, Cathy Graham, Beth-Ann Lesnikoski, Kathryn Wagner, June Lee, Julian Berrocal, Amy M. Truitt,
Erin Yoder, Lisa Blumencranz, Bastiaan van der Baan, William Audeh, FLEX Investigators’ Group
Background:
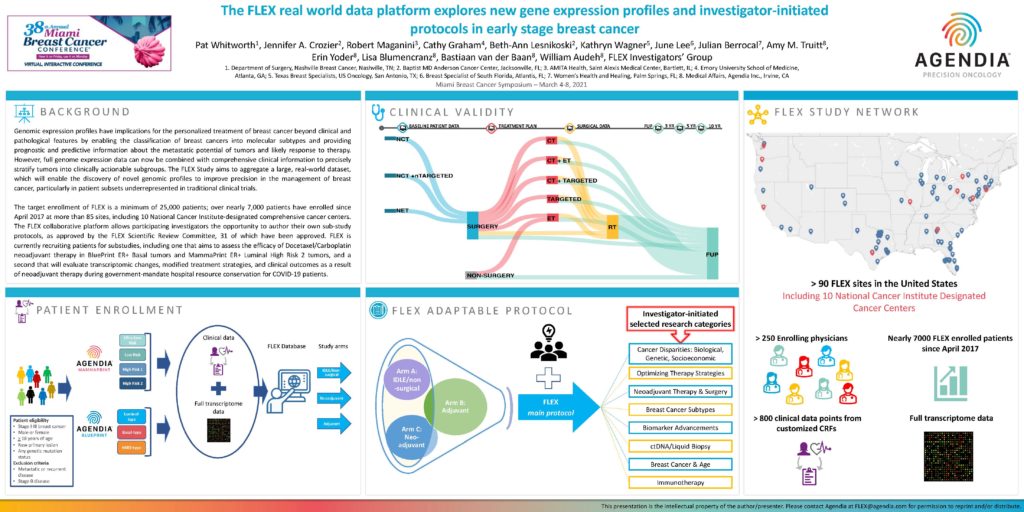
Genomic expression profiles have implications for the personalized treatment of breast cancer beyond clinical and pathological features by enabling the classification of breast cancers into molecular subtypes and providing prognostic and predictive information about the metastatic potential of tumors and likely response to therapy. However, full genome expression data can now be combined with comprehensive clinical information to precisely stratify tumors into clinically actionable subgroups. The FLEX Study aims to aggregate a large, real-world dataset, which will enable the discovery of novel genomic profiles to improve precision in the management of breast cancer, particularly in patient subsets underrepresented in traditional clinical trials.
The target enrollment of FLEX is a minimum of 25,000 patients; over nearly 7,000 patients have enrolled since April 2017 at more than 85 sites, including 10 National Cancer Institute-designated comprehensive cancer centers. The FLEX collaborative platform allows participating investigators the opportunity to author their own sub-study protocols, as approved by the FLEX Scientific Review Committee, 31 of which have been approved. FLEX is currently recruiting patients for substudies, including one that aims to assess the efficacy of Docetaxel/Carboplatin neoadjuvant therapy in BluePrint ER+ Basal tumors and MammaPrint ER+ Luminal High Risk 2 tumors, and a second that will evaluate transcriptomic changes, modified treatment strategies, and clinical outcomes as a result of neoadjuvant therapy during government-mandate hospital resource conservation for COVID-19 patients.