AUTHORS:
Robert Maganini, Pond Kelemen, Jennifer A. Crozier, Gordan Srkalovic, Adam Brufsky, Mehran Habibi, Ian Grady, Nina D’Abreo, Michelle Bolner, Sarah Untch, Erin Yoder, Heather M. Kling, William Audeh, Bastiaan van der Baan FLEX Investigators Group
DESCRIPTION:
The FLEX Real World Data Platform Explores New Gene Expression Profiles and Investigator-Initiated Protocols in Early Stage Breast Cancer
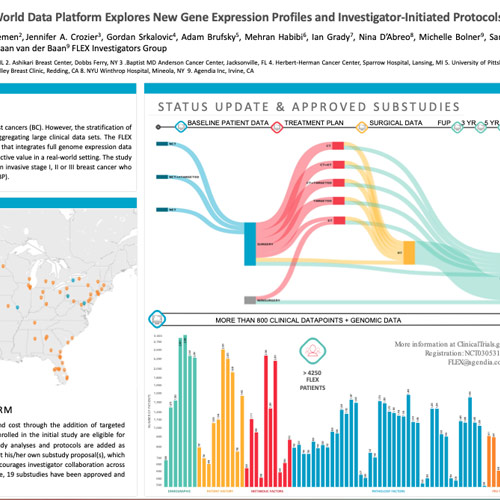
Genomic signatures have revolutionized the identification and treatment of breast cancers (BC). However, the stratification of breast cancers into actionable subtypes has been limited by the difficulty in aggregating large clinical data sets. The FLEX Registry was designed to operate as a multicenter, large-scale, prospective study that integrates full genome expression data with clinical data to investigate new gene signatures with prognostic and/or predictive value in a real-world setting. The study will enroll a minimum of 10,000 patients aged ≥18 years with histologically proven invasive stage I, II or III breast cancer who sign informed consent and receive MammaPrint (MP) with or without BluePrint (BP).
Read more: Miami Breast 2020 FLEX Trials in Progress