
Optimize Endocrine Therapy Duration in women with HR+/HER2-negative early breast cancer
Optimize Endocrine Therapy Duration in women with HR+/HER2-negative early breast cancer
> May REDUCE ET to <5 years in the event of intolerance1,2 (MP UltraLow)
> Show benefit from 10 years of EET3,4 (MP Low)
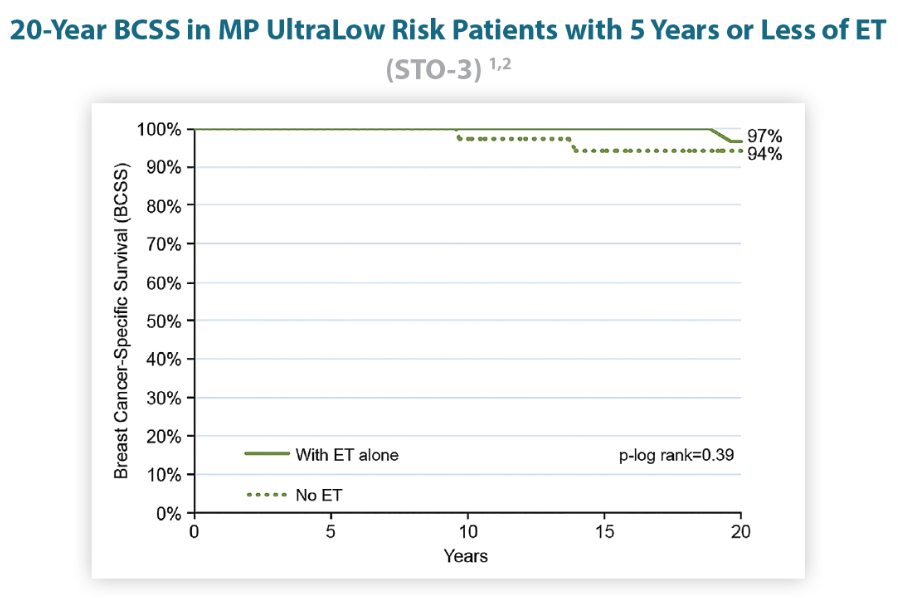
MammaPrint UltraLow Risk patients may consider <5 years of endocrine therapy1,2
Identifying MammaPrint UltraLow LN0 postmenopausal patients who have excellent 20-year BCSS with only 2 years of ET can prevent potential overtreatment and enable excellent outcomes with less toxic effects.1
*MammaPrint UltraLow is included in the 2023 NCCN Guidelines ‘Gene Expression Assays for Consideration of Adjuvant Systemic Therapy’ Visit www.nccn.org to learn more
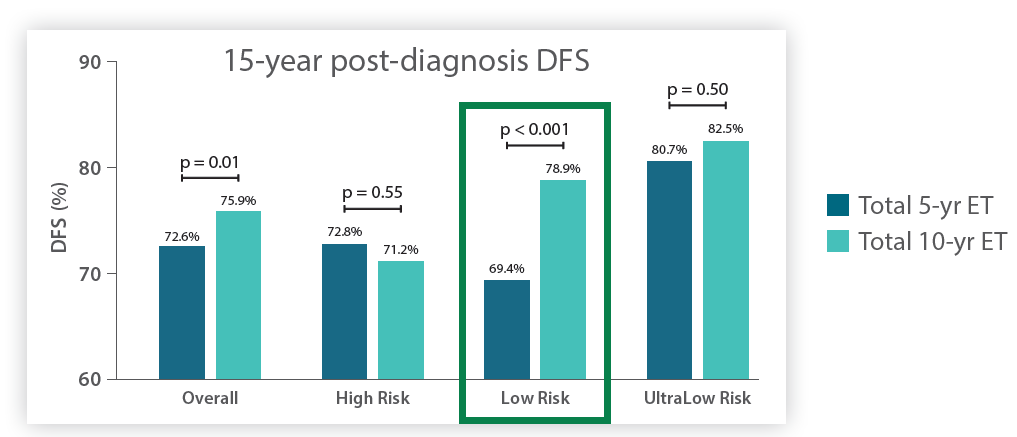
MammaPrint predicts benefit from extended endocrine therapy
in postmenopausal Low Risk patients.3,4
in postmenopausal Low Risk patients.3,4
Recent data demonstrate that MammaPrint High 2, BluePrint Luminal B, is predictive of benefit from AC-T therapy, with a higher 3-year RFS rate than with TC therapy, alone.
*Data shown in bar chart is from NSABP-B42
Dive deeper into the data with our Medical team


