AUTHORS: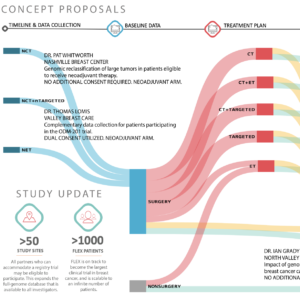
Adam M. Brufsky, Jennifer A. Crozier, Ian Grady, Thomas Lomis, Pat Whitworth, Esther Rehmus, Gordon Srkalovic, Laura Lee, Peter Blumencranz, Paul Baron, Blanche Mavromatis, Sarah Untch, Lisa Blumencranz, Tina Treece, Erin Yoder, William Audeh.
DESCRIPTION:
MammaPrint, BluePrint, and Full-genome Data Linked with Clinical Data to Evaluate New Gene EXpression Profiles (FLEX)
Genomic signatures are revolutionizing the definition, identification, and treatment of breast cancer subtypes. The ability of genomic signatures to enable fine grained stratification of breast cancers to the granular disease level is still generally untested because of the difficulties in aggregating large clinical data sets. In order to stratify breast cancers into actionable subtypes both the full genome data and relevant clinical data must be collected for patients at scale. Traditional one-drug, one-test, one-trial models are slow, arduous, and yield low sample sizes and high objective failures by phase III trials.
The study will enroll a minimum of 10000 patients aged =18 years with histologically proven invasive stage I-III breast cancer who signed informed consent. Enrollment began April 2017 and 623 patients have been enrolled as of June 2018.
Read more: 2018 SABCS – FLEX Trials in Progress Poster



